Abstract

Background MF is characterized by splenomegaly, anemia, debilitating symptoms and bone marrow (BM) fibrosis. Rux is the current standard of care improving symptoms and splenomegaly but with little impact on the malignant clone and BM fibrosis. IFNa can reduce mutant allele burden and fibrosis but is poorly tolerated in highly symptomatic patients (pts). The RUXOPEG study was designed to assess the efficacy and safety of different doses combinations of Rux + pegylated IFNa 2a in both JAK inhibitor and IFNa-naïve MF pts (NCT02742324).
Methods RUXOPEG is a multi-center Bayesian Phase 1/2 adaptive trial. Phase 1 (P1) evaluated 9 possible combinations of doses of Rux, and IFNa (10, 15 and 20 mg BID, and 45, 90 and 135 mcg/week, respectively) and included 6 cohorts of 3 pts. Phase 2 (P2) randomized the 2 best dose combinations selected from P1. Primary tolerance criterion was the occurrence of dose limiting toxicities (DLT) within 45 days in P1. Primary efficacy criterion was >50% reduction in spleen length (SL) by palpation within 24 weeks (W). Secondary objectives included molecular response, quality of life and symptoms evolution, reduction of BM fibrosis, event-free and overall survival. Key inclusion criteria: diagnosis of MF (WHO), Intermediate (Int) or high risk (HR) (IPSS), need of active therapy. Key exclusion criteria: prior treatment with Rux or IFNa, eligibility for stem cell transplantation, autoimmune disease, history of depression.
Enrolment in P1 was completed in Nov 2018 and in P2 in Nov 2020. We report the data collected after last pt last visit after 12 months in the study (Nov 2021).
Results A total of 37 pts (18 in P1, 19 in P2) were included from 6 centers. Median (range) age was 63.5 years (37-71), 21 had primary MF, 16 post ET or post PV MF. Median (range) SL was 9 cm (0-20) by palpation and 19.5 cm (10.6-30) by imaging. Mean (range) hemoglobin was 12.2 g/dL (7.8-16.2), WBC 12.2 G/L (3.5- 36.1), platelets 372 G/L (150-1304). IPSS at inclusion: Int-1 in 23, Int-2 or HR in 14 pts. Mean number of mutations (mut) per pt was 3.4.; 21 pts had JAK2V617F, 11 CALR and 4 MPL mut; NGS identified 90 additional mut in 17 genes in 33 pts, including mut in HR genes: ASXL1 (n= 21), EZH2 (5), SRSF2 (2), IDH1 (1), and TP53 (5).
Safety: No DLT occurred among the 18 pts included in 6 cohorts of P1 (primary safety criterion), and 19 pts were randomized between the 2 dose combinations selected for P2: IFNa 135 mcg/w + Rux 15 (level 6, 9 pts) or 20 (level 9, 10 pts) mg BID. Over 24W, a total of 363 AEs (59% gr1, 33% gr2, 7.5% gr3, 0.5% gr4) including 6 SAE (2 skin cancers, 2 anemias, 1 urinary infection, 1 Raynaud's phenomenon) were reported. Only 2.5% of them resulted in treatment interruption. Most frequent AEs were anemia (18.7%), thrombocytopenia (13.7%), GI (7%), musculoskeletal (6.8%) and asthenia (6.6%). During the 12-month period of the study, 1 patient died of AML transformation after 10.4 months.
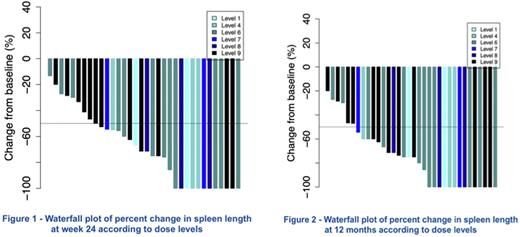
Efficacy: Primary efficacy endpoint (reduction by at least 50% of SL within 24 W) was reached in 70% (IC95%: 53;84) of pts (26/37 pts in ITT, 67% in P1, 74% in P2) with a clear decrease in SL at W24 (median 3.5 cm by palpation, range 0-12) (Fig 1), maintained at 12 months (median SL of 3 cm, range 0-12) (Fig 2) including complete resolution of palpable splenomegaly in 38% of pts. The best response rate (83%) was seen in patients receiving dose level 6. JAK2V617F allele burden decreased from a median (range) of 84% (23-96) at baseline to 65% (16-95), and 53% (16-92) after 6, and 12 months. Genotyping of progenitor-derived colonies performed in 10 patients showed that the combination of drugs clearly targets in 12 months committed (CD34+CD38+), immature (CD34+CD38-) and HSC-enriched progenitors (CD90+CD34+CD38-) carrying homozygous or heterozygous JAK2V617F. BM biopsies at inclusion and after 12 months are currently centrally reviewed (preliminary results show reduction of BM cellularity and grade of fibrosis in selected pts), and results of additional exploratory endpoints including evolution of clonal architecture with single cell and NGS sequencing, cytokine array and changes in immune effector cells will be presented.
Conclusion RUXOPEG is the first study to formally assess the safety and efficacy of Rux + pegylated IFNa 2a combination in patients with MF never exposed to either drug before. Final results show that the combination provided high rates of important reductions in SL and JAK2V617F allele burden due to selective targeting of mutated progenitors.
Disclosures
Kiladjian:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; BMS: Consultancy; AOP Orphan: Membership on an entity's Board of Directors or advisory committees. Ianotto:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Soret:Novartis: Consultancy. Boyer perrard:Novartis: Honoraria. Plo:Incyte: Research Funding.
OffLabel Disclosure:
Off label use of pegylated interferon alpha 2a in patients with myelofibrosis in a clinical trial.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal